Donations of blood and its components (red cells, platelets, and plasma) facilitate a wide range of essential, often life-saving treatments. Blood transfusions are essential when dealing with trauma or major surgery, and are often needed in cancer management, or to treat inherited chronic blood diseases such as Thalassaemia.
Donated plasma, a component of blood, can also be used to manufacture medicinal products like immunoglobulins or clotting factors. The manufacture of these products is subject to pharmaceutical legislation, while the donation, collection and testing of plasma is regulated by blood legislation.
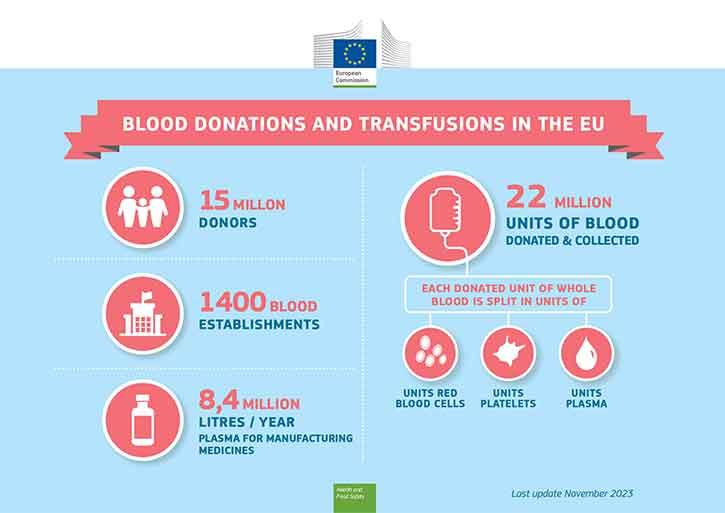
The availability of blood, and blood components, is dependent on citizens' willingness to donate blood. Most people who are in good health can donate blood.
The European Commission provides funding for Actions in the area of SoHO mainly in the form of projects or joint actions with national authorities. Actions aim at supporting the EU mandate on safety and quality, but can also serve to promote other policy priorities, such as improving the availability of SOHO or the efficiency of the health systems that support donation and supply.
Legislation
The legal framework defining the quality and safety standards for blood and its components is set out in Directive 2002/98/EC, also referred to as the European Blood Directive. It covers all steps in the transfusion process from donation, collection, testing, processing, and storage to distribution.
To help implement this main act, the European Commission proposed and adopted, in close collaboration with EU national authorities, the following additional implementing acts:
- Commission Directive 2004/33/EC on the technical requirements for blood and blood donation
- Commission Directive 2005/61/EC on the traceability requirements and notification responsibilities in case of serious adverse reactions and events
- Commission Directive 2005/62/EC that sets out Community standards and specifications relating to the quality system for a blood bank
Commission Directives 2009/135/EC, 2011/38/EU, 2014/110/EU, 2016/1214 address some further specific technical requirements.
It is important to note that EU countries can always choose to apply more stringent rules to the quality and safety of blood and blood products than those outlined above.
Following an evaluation of the EU legislation on blood and tissues and cells, published in 2019, the Commission has proposed a revision of this legislation which was adopted on 14 July 2022.
Coordination
National competent authorities are responsible for the implementation of EU legislation. The European Commission holds regular meetings with them to facilitate communication, to exchange best practices, and to reach a common understanding on the implementation of the Directives.
Periodic surveys, completed by the competent authorities, allow the Commission to draft reports on the state of play of implementation of the legislation.






