While organ transplantation is increasingly used as medical treatment, the main factor limiting its application remains the shortage of available organs.
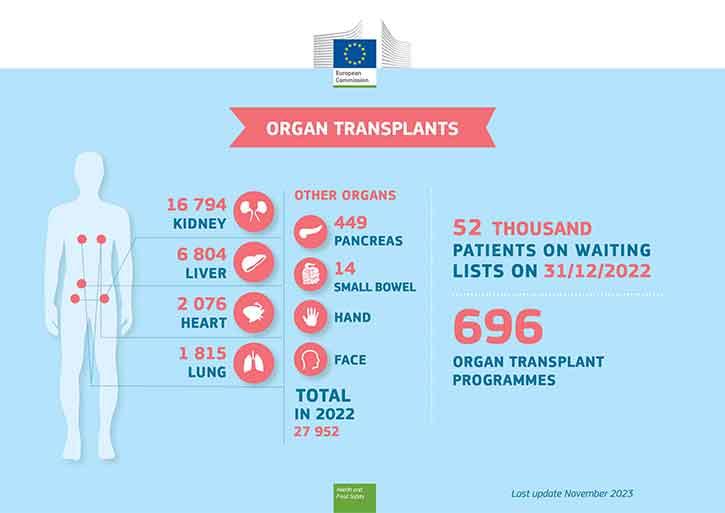
Kidneys are the most frequently transplanted organ and help patients with end-stage kidney disease. Other frequently transplanted organs include livers, lungs and hearts, while the small bowel and the pancreas can also be transplanted, and new types of transplantation are continuously being developed.
All organs can be donated after death, while living donors can donate a kidney as well as a part of their liver.
The European Commission provides funding for Actions in the area of SoHO mainly in the form of projects or joint actions with national authorities. Actions aim at supporting the EU mandate on safety and quality, but can also serve to promote other policy priorities, such as improving the availability of SOHO or the efficiency of the health systems that support donation and supply.
The European Commission has been supporting National Competent Authorities (NCA) by facilitating the sharing of information among them, including guidance from the European Centre for Disease Prevention and Control (ECDC), in particular during the COVID-19 crisis. The European Commission plans further EC actions to continue supporting the Organs sector, mainly on two dimensions:
- Support EU Countries and comply with the reporting obligation set in the Directive.
- Support the sector via EU4Health grants and financial support to expert bodies.
Legislation
The legal framework defining the standards for organ transplantation is set out in Directive 2010/53/EU, also referred to as the European Organs Directive.
The Directive lays down the quality and safety standards for organs. It covers all steps in the transplant process from donation, through procurement, testing, handling to distribution.
To help implement this basic act, the Commission proposed and adopted, in close collaboration with EU national authorities, Commission Directive 2012/25/EU regarding the information procedures for exchange of human organs intended for transplantation, between EU countries.
The Commission's proposal for the Organs Directive was accompanied by an Impact Assessment. Resolution 2007/2210, Council Conclusions No 15332/07 SAN on organ donation and transplantation, the Communication from the Commission COM (2007) 275 and the Consultation on Organ Donation and Transplantation that lay the groundwork for the existing legislation.
Guidelines
The European Commission collaborates closely with expert bodies such as the Council of Europe (CoE) and the European Centre for Disease Prevention and Control (ECDC) in the development of practical guidelines that support transplant centres and procurement organisations with the implementation of this binding legislative framework.
- The Council of Europe (CoE) regularly reviews and updates technical requirements in its Guide to the quality and safety of Organs for Transplantation. In addition, it has published a Recommendation on organ trafficking.
- The ECDC prepares risk assessments and preparedness plans whenever epidemiological outbreaks are of relevance for blood, tissues, cells and organs. Most recently ECDC has provided input on:
- Ebola
- Hepatitis A
- West Nile Virus
- Zika
Coordination and implementation
National Competent Authorities (CAs) are in charge of the implementation of the requirements established in EU legislation. The European Commission holds regular meetings with them to facilitate communication and the exchange of best practices, as well as to reach a common understanding on the implementation of the Directives.
Periodic surveys, completed by the CAs, allow the European Commission to draft reports on the current implementation status of the legislation.
Occasionally, the EU National Competent Authorities adopt a statement on a topic of common interest or concern:
- Statement on Organ Donation and Transplantation and the COVID-19 pandemic
- Statement on a proposed concept of Global Kidney Exchange
Action plan
The European Action Plan on Organ Donation and Transplantation (2009-2015): Strengthened Cooperation between Member States assists EU countries in addressing the shortage of organs, enhancing transplant systems and improving the quality and safety of transplant products. It has set a common direction for EU countries to strengthen their national transplant activities and served as the basis for many EU-funded actions. Over its period of implementation, 2009-2015, EU countries increased activities by 4,600 additional transplants per year.